This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Absolute zero in the quantum computer: Formulation for the third law of thermodynamics
When many quantum particles interact, complex systems can be formed. And this complexity allows reaching a temperature of absolute zero—at least in principle.
The absolute lowest temperature possible is -273.15°C. It is never possible to cool any object exactly to this temperature—one can only approach absolute zero. This is the third law of thermodynamics.
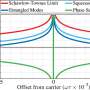
In a study published in PRX Quantum, a research team at TU Wien (Vienna) has now investigated the question: How can this law be reconciled with the rules of quantum physics? They succeeded in developing a "quantum version" of the third law of thermodynamics: Theoretically, absolute zero is attainable. But for any conceivable recipe for it, you need three ingredients: Energy, time and complexity. And only if you have an infinite amount of one of these ingredients can you reach absolute zero.
Information and thermodynamics: An apparent contradiction
When quantum particles reach absolute zero, their state is precisely known: They are guaranteed to be in the state with the lowest energy. The particles then no longer contain any information about what state they were in before. Everything that may have happened to the particle before is perfectly erased. From a quantum physics point of view, cooling and deleting information are thus closely related.
At this point, two important physical theories meet: Information theory and thermodynamics. But the two seem to contradict each other. "From information theory, we know the so-called Landauer principle. It says that a very specific minimum amount of energy is required to delete one bit of information," explains Prof. Marcus Huber from the Atomic Institute of TU Wien. Thermodynamics, however, says that you need an infinite amount of energy to cool anything down exactly to absolute zero. But if deleting information and cooling to absolute zero are the same thing—how does that fit together?
Energy, time and complexity
The roots of the problem lie in the fact that thermodynamics was formulated in the 19th century for classical objects—for steam engines, refrigerators or glowing pieces of coal. At that time, people had no idea about quantum theory. If we want to understand the thermodynamics of individual particles, we first have to analyze how thermodynamics and quantum physics interact—and that is exactly what Marcus Huber and his team did.
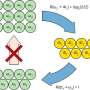
"We quickly realized that you don't necessarily have to use infinite energy to reach absolute zero," says Marcus Huber. "It is also possible with finite energy—but then you need an infinitely long time to do it." Up to this point, the considerations are still compatible with classical thermodynamics as we know it from textbooks. But then the team came across an additional detail of crucial importance:
"We found that quantum systems can be defined that allow the absolute ground state to be reached even at finite energy and in finite time—none of us had expected that," says Marcus Huber. "But these special quantum systems have another important property: they are infinitely complex." So you would need infinitely precise control over infinitely many details of the quantum system—then you could cool a quantum object to absolute zero in finite time with finite energy. In practice, of course, this is just as unattainable as infinitely high energy or infinitely long time.
Erasing data in the quantum computer
"So if you want to perfectly erase quantum information in a quantum computer, and in the process transfer a qubit to a perfectly pure ground state, then theoretically you would need an infinitely complex quantum computer that can perfectly control an infinite number of particles," says Marcus Huber. In practice, however, perfection is not necessary—no machine is ever perfect. It is enough for a quantum computer to do its job fairly well. So the new results are not an obstacle in principle to the development of quantum computers.
In practical applications of quantum technologies, temperature plays a key role today—the higher the temperature, the easier it is for quantum states to break and become unusable for any technical use. "This is precisely why it is so important to better understand the connection between quantum theory and thermodynamics," says Marcus Huber. "There is a lot of interesting progress in this area at the moment. It is slowly becoming possible to see how these two important parts of physics intertwine."
More information: Philip Taranto et al, Landauer Versus Nernst: What is the True Cost of Cooling a Quantum System?, PRX Quantum (2023). DOI: 10.1103/PRXQuantum.4.010332
Journal information: PRX Quantum
Provided by Vienna University of Technology