Credit: Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202310983
To ensure that vaccines provide strong and lasting immunization, it is often necessary to supplement the actual vaccine (antigen) with additives that stimulate the immune system: adjuvants. Today, only a few substances have been approved for use as adjuvants.
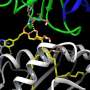
In the journal Angewandte Chemie International Edition, a research team has now introduced a spectrum of potential adjuvants. They started with the immune stimulant α-glactosyl ceramide (α-GalCer) and synthesized many different variants from a set of four building blocks.
α-GalCer is a synthetic glycolipid (a compound made from fat and sugar building blocks) based on similar compounds found in sea sponges. It binds to CD1-d, a special receptor on antigen-presenting cells. This activates certain immune cells and induces secretion of cytokines that stimulate the immune system. In this way, this substance boosts the immune response, assists in the battle against pathogens and tumor cells, and reduces autoimmune reactions.
Among the newly synthesized α-GalCer analogs, the team led by Berhnard Westermann, Daniel G. Rivera, and Carlos A. Guzmán from the Leibniz Institute of Plant Biochemistry (Halle/Saale) and the Helmholtz Center for Infection Research (Braunschweig) identified a number of compounds that have significantly better and/or somewhat different activity.
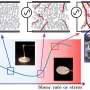
The key to their success was the use of a special reaction for the synthesis of the α-GalCer analogs: In a reaction known as the Ugi four-component reaction, the target molecules are assembled in one step from four individual building blocks. The team varied these four components broadly in a combinatorial method and synthesized a collection of different α-GalCer derivatives. In particular, they used a functional group (N-substituent of the amide bond) that had not been employed in the derivatization of α-GalCer before. This allowed the team to introduce many different additional functionalities into their α-GalCer analogs.
This strategy led to the discovery of compounds that trigger stronger antigen-specific T-cell stimulation and higher antibody reaction when they are administered to mice together with a model antigen, either by injection or through the nasal mucosa. In addition, various functionalized α-GalCer analogs demonstrated stronger adjuvant activity in vitro and in animal studies that an α-GalCer previously optimized (conjugated with polyethylene glycol) for this purpose.
Interestingly, some of the new analogs showed somewhat different effects on the immune system, making it possible to elicit differently balanced immune responses through controlled variation of the derivatization. This could make it possible to develop adjuvants that can be tailored precisely to the requirements of the pathogens in question. In addition, it may be possible to introduce an additional binding site through which the antigen could be bound directly to the adjuvant without compromising its effect—a requirement for the development of self-adjuvating vaccines.
More information: Yanira Méndez et al, Diversification of a Novel α‐Galactosyl Ceramide Hotspot Boosts the Adjuvant Properties in Parenteral and Mucosal Vaccines, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202310983
Journal information: Angewandte Chemie International Edition
Provided by Wiley